Background: Bispecific T-cell engagers (BTEs) are a novel class of drugs used to treat hematological malignancies that link endogenous tumor antigens to CD3 on the T-cell receptor, priming circulating T-cells to effectively target tumor cells. Although chimeric antigen receptor T-cell therapies, a closely related class, have been associated with cardiovascular toxicity, little is known about the cardiovascular toxicity of BTEs. As early clinical trials may have been underpowered for the detection of cardiovascular adverse events (CVAEs), we conducted a large-scale post-marketing surveillance study to identify safety signals that may have been missed.
Methods: Leveraging the US Food and Drug Administration's Adverse Events Reporting System (FAERS), we identified adverse event (AE) reports in patients who received BTEs from October 2014 to March 2023. Eligible therapies included Blinatumomab (CD3/CD19 bispecific antibody) and Teclistamab (CD3/BCMA bispecific antibody). The primary outcome of the study was the frequency and fatality rates of CVAEs associated with BTEs. CVAEs included bleeding, hypotension or shock, thromboembolic disease, heart failure, conduction abnormalities (including tachyarrhythmia, bradyarrhythmia, QT-prolongation, and premature contractions), myocarditis, pericarditis, sudden death, and vasculitis. Secondary outcomes included the frequency and fatality rates of cytokine release syndrome (CRS), neurotoxicity (seizures, altered mental status [AMS], speech disorders, gait disorders, and tremors), and infections. We used multivariable logistic regression models, adjusting for age, sex, and disease status, to calculate adjusted reporting odds ratios (RORs). RORs represent the likelihood of reporting a given AE with BTEs compared to reporting the same AE with all other drugs in the database. Mortality rates for each AE are also reported.
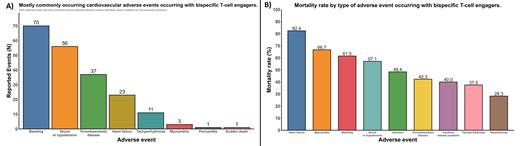
Results: Overall, 1,181 cases were reported to FAERS for BTE-associated AEs. Among these, 148 (12.5%) involved CVAEs. The most common CVAEs (non-exclusive) were bleeding (70 events), hypotension or shock (56 events), thromboembolic disease (37 events), heart failure (23 events), and tachyarrhythmias (11 events). Less common CVAEs included myocarditis (3 events), pericarditis (1 event), sudden death (1 event), and vasculitis (1 event) (Figure 1A). Of these, BTEs as a class were disproportionately associated with shock or hypotension (ROR: 1.37 [95% CI: 1.03 to 1.80]).
Blinatumomab was associated with disproportionately high rates of thromboembolic disease (ROR: 1.48 [95% CI: 1.05 to 2.08]), particularly disseminated intravascular coagulation (DIC; ROR: 4.67 [95% CI: 2.85 to 7.63]). Teclistamab was associated with disproportionately high rates of myocarditis (ROR: 35.79 [95% CI: 10.47 to 122.41]), QT prolongation (ROR: 9.85 [95% CI: 1.33 to 73.08]), and sudden death (ROR: 8.62 [95% CI: 1.16 to 63.99]).
Compared to non-cardiovascular AEs, CVAEs were associated with a 92% relative increase in mortality (25.9% vs 50.7%; risk ratio: 1.92 [95% CI: 1.53 to 2.42]). Among CVAEs, the highest mortality rates were reported for heart failure (82.4%), myocarditis (66.7%), bleeding (61.5%), shock or hypotension (57.1%), thromboembolic disease (42.3%), and tachyarrhythmias (37.5%) (Figure 1B).
Non-cardiovascular AEs such as neurotoxicity (322 events), infection (277 events), and CRS (190 events) were reported more frequently but had lower mortality rates (28.3%, 48.4%, and 40.0% respectively). Blinatumomab was associated with disproportionately high rates of CRS (ROR: 6.54 [95% CI: 5.41 to 7.92]) and neurotoxicity (ROR: 2.94 [95% CI: 2.56 to 3.39]). Neurotoxicity included AMS (ROR: 2.47 [95% CI: 2.01 to 3.04]), seizures (ROR: 2.81 [95% CI: 2.09 to 3.77]), speech disorders (ROR: 5.72 [95% CI: 4.16 to 7.85]), and tremors (ROR: 6.94 [95% CI: 5.15 to 9.34]). Teclistamab was associated with CRS (ROR: 134.35 [95% CI: 74.45 to 242.47]), infections (ROR: 6.83 [95% CI: 4.63 to 10.07]), and neurotoxicity (ROR: 2.75 [95% CI: 1.53 to 4.94]).
Conclusion: In the first large-scale post-marketing surveillance study of BTEs, CVAEs were not uncommon and were more likely to be fatal compared to non-CVAE. Heart failure and myocarditis in particular may portend worse outcomes. These safety signals warrant clinical vigilance and further investigation of their mechanisms, optimal surveillance strategies, and treatment options.
Disclosures
Epperla:Beigene: Research Funding, Speakers Bureau; Incyte: Speakers Bureau; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Merck: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; ADC Therapeutics: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Lilly: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal